Camurus

Acromegaly is a rare condition where the body produces too much growth hormone, causing body tissues and bones to grow more quickly. Over time, this leads to abnormally large hands and feet, and a wide range of other symptoms.
To increase the awareness of the Camurus clinical program amongst acromegaly patients who may be interested in participating in the trials. W was asked to develop a simple, yet informative patient website to motivate patients with acromegaly to contact a clinical trial center in the US to learn more about participating in the clinical trials.
Communicating to patients is a complex process, not only from a regulatory perspective. But also, from the perspective of ensuring that both the language and content are relevant to a patient, e.g., using terms, words, and phrases that the patient will be familiar with. Based on this understanding, W in collaboration with Camurus developed a simple sitemap, to capture the key information that a patient would need to know about the Camurus clinical program in the US.
To accompany the patient site, W also created a password protected website with relevant tools and information for clinical trial investigators involved in recruiting patients into these studies.
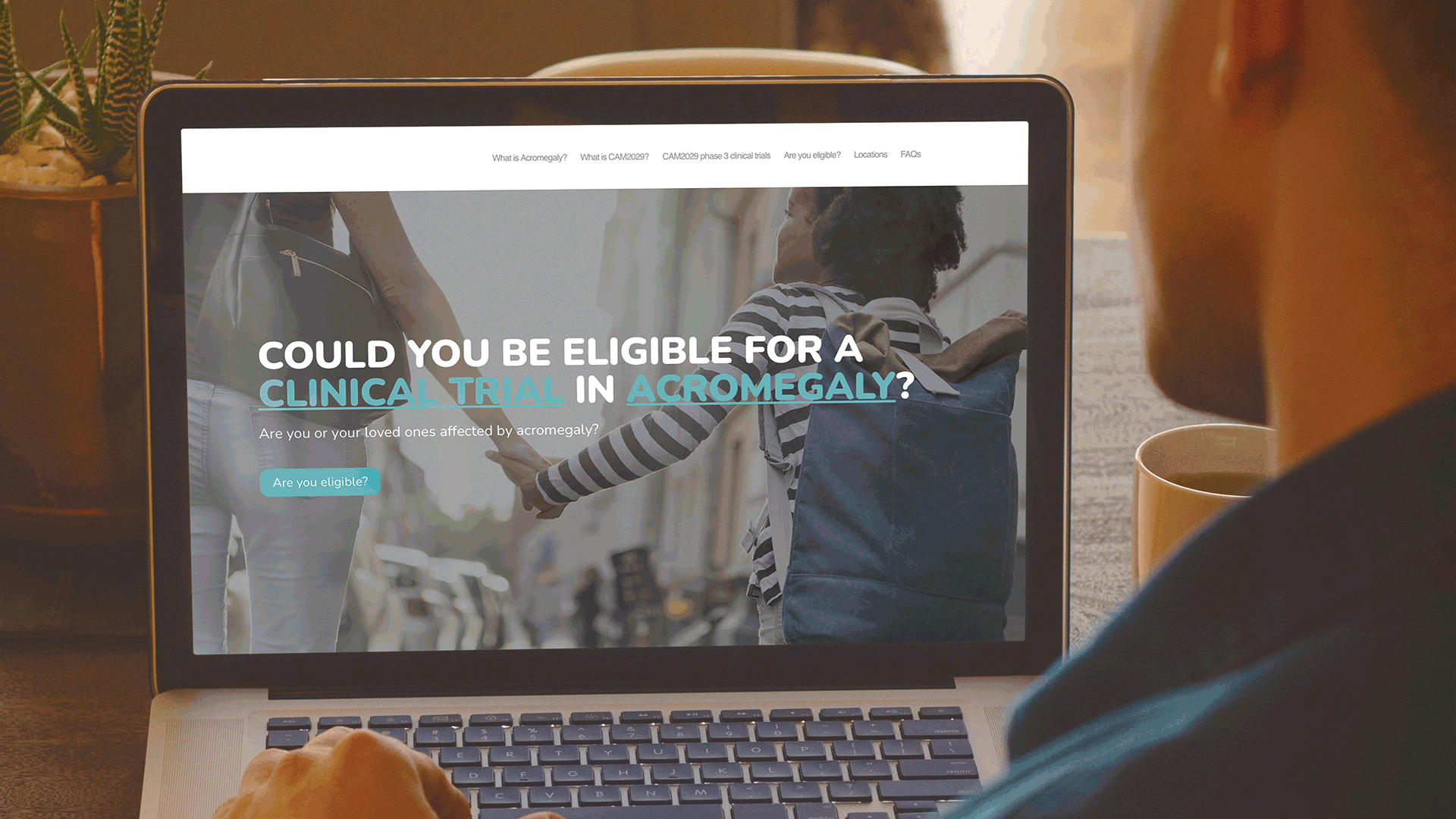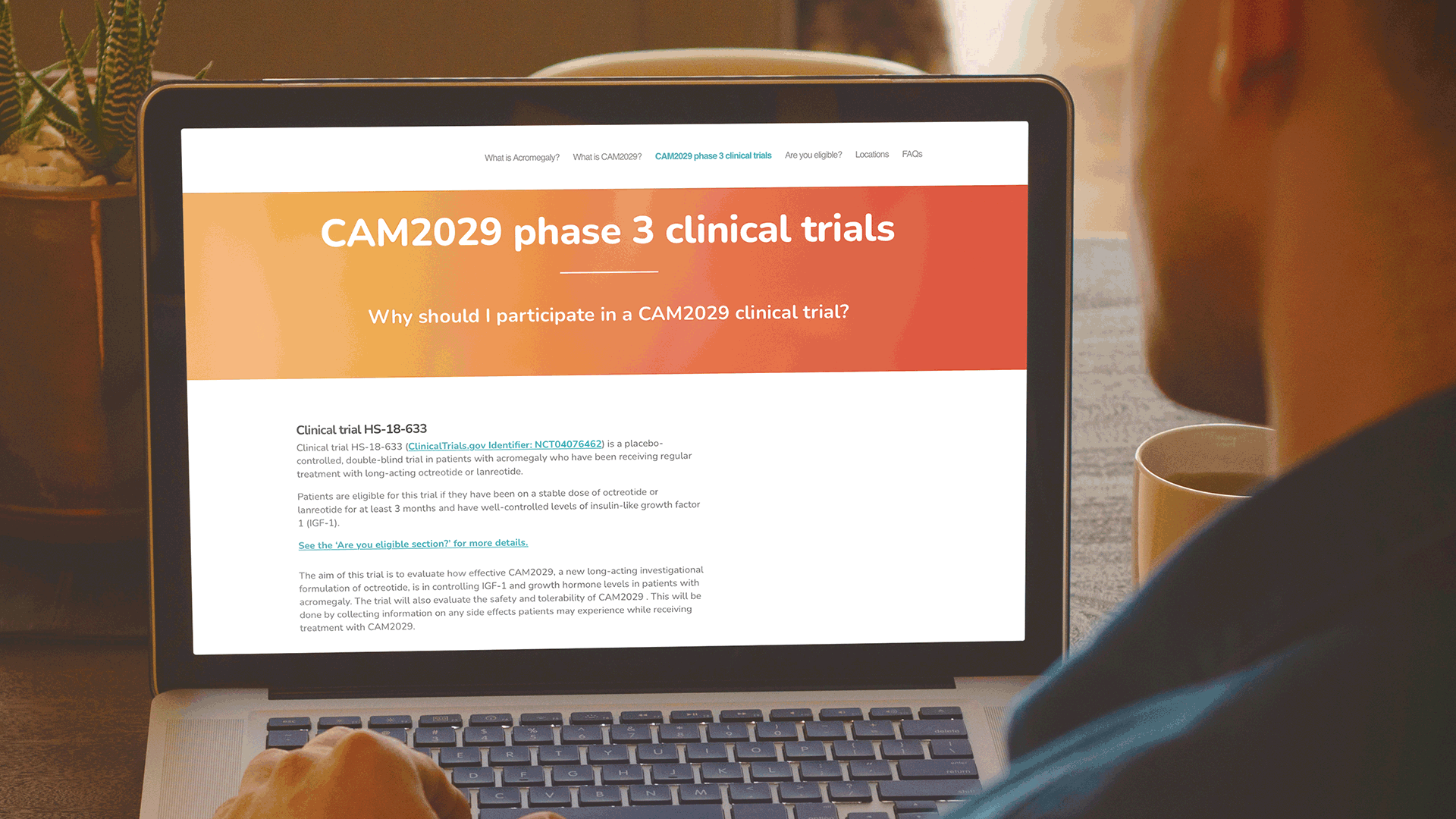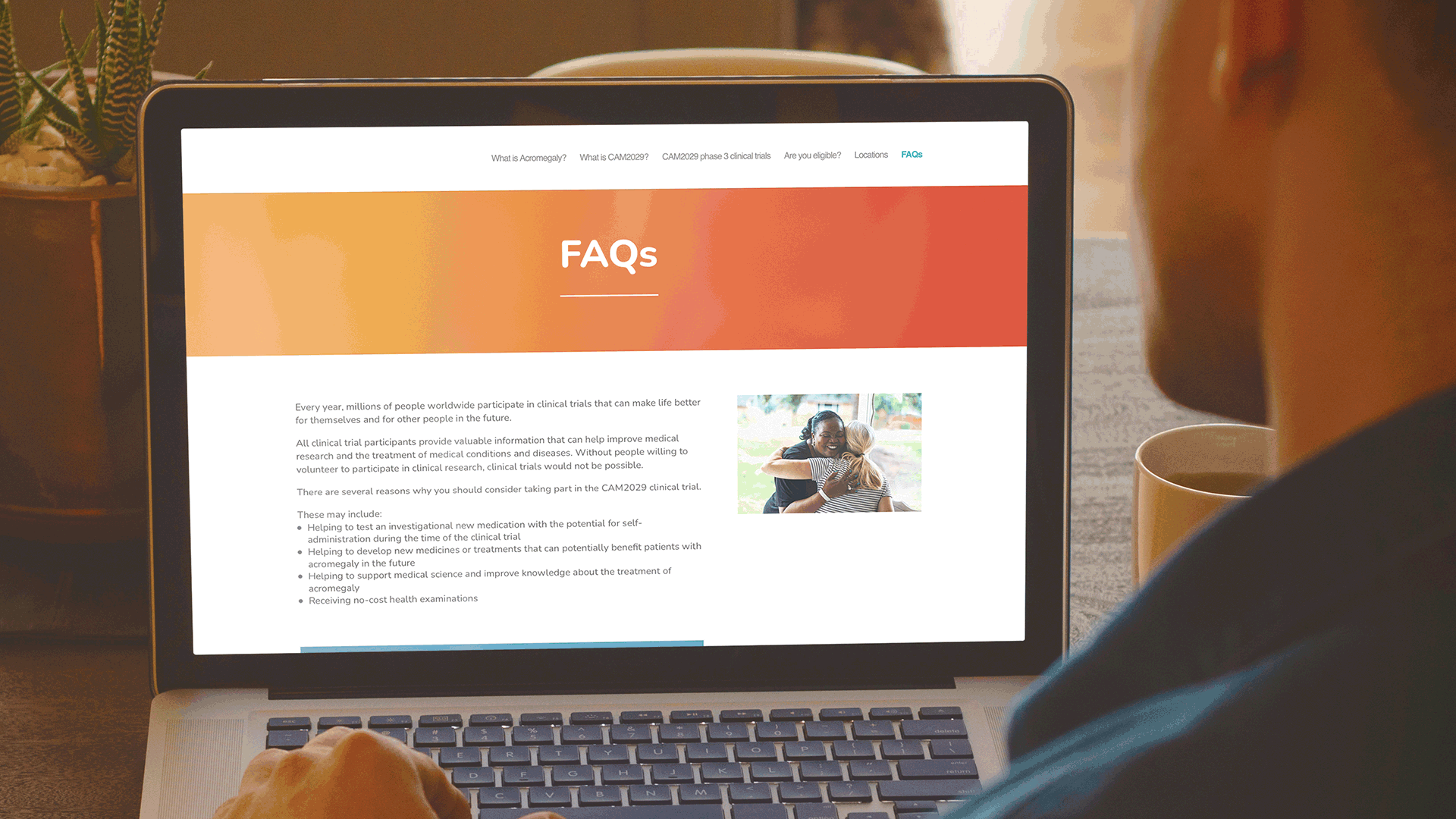
These websites have been very well received by the clinical development team at Camurus and are being actively promoted by Camurus during discussions with all clinical trial investigators. Both websites were also highlighted in the recent Camurus Acromegaly clinical trials newsletter.
Get news and inspiration from W straight to your inbox – sign up for our newsletter!